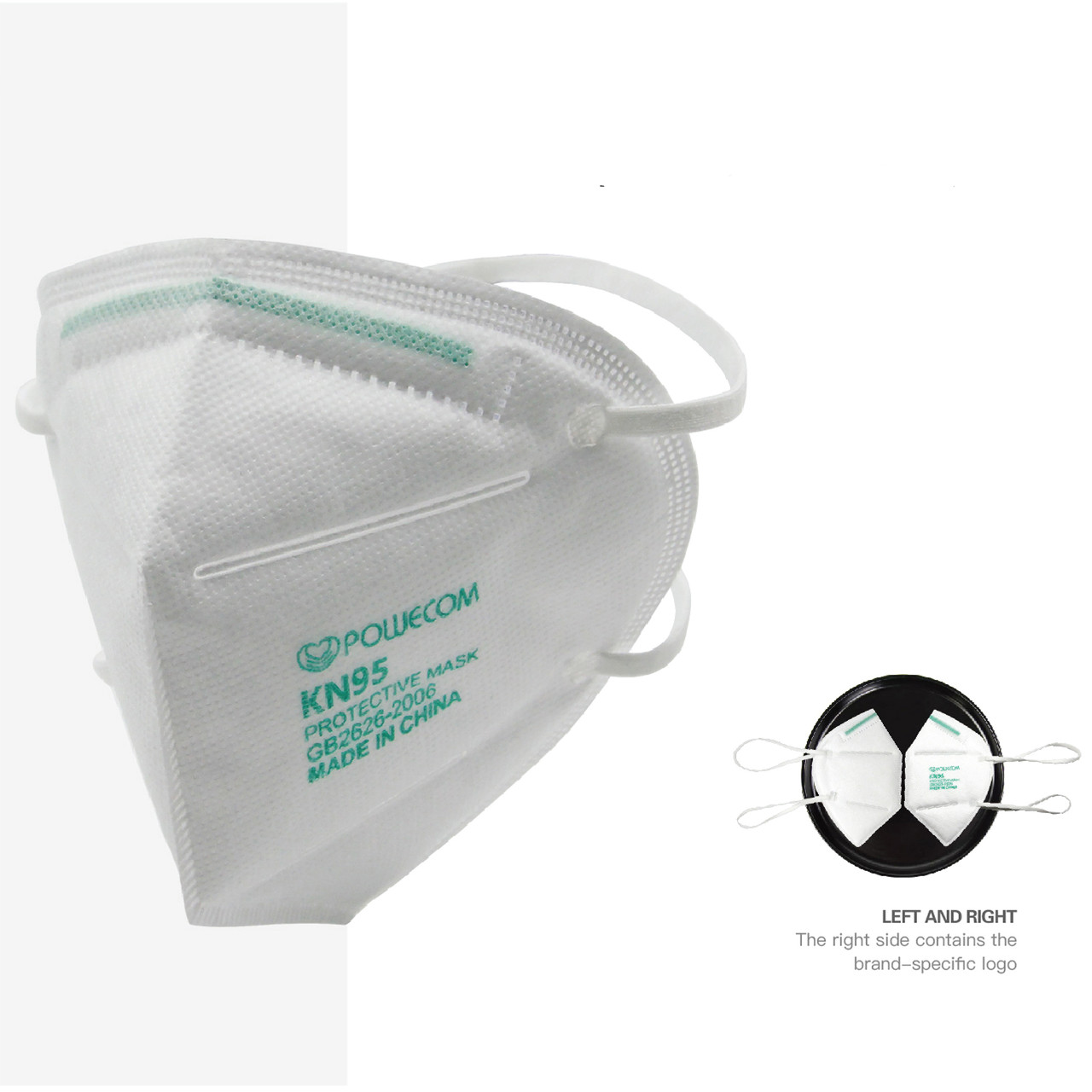
Product Description
The headband style Powecom KN95 respirator face mask, shipping from stock from our Mt. Vernon, NY warehouse, is made of a multi-layer filtration system of non-woven breathable fibers and is utilized globally. This specific model is the headband style KN95 from Guangzhou Powecom Labor Insurance Supplies Co., LTD., and is FDA authorized for use in healthcare settings by HCP during the COVID-19 public health emergency: https://www.fda.gov/media/136664/download. FDA authorized respirators should be used in accordance with CDC recommendations. For the most current CDC recommendations on optimizing respirator use, please visit the CDC webpage: Strategies for Optimizing the Supply of N95 Respirators.
Guangzhou Powecom Labor Insurance Supplies Co., Ltd., the manufacturer of our FDA authorized KN95 respirator mask, has designated Bona Fide Masks™, a part of the Ball Chain Mfg. Co., Inc., family, as their premier distributor in the United States.
FDA Appendix A, listing non-NIOSH approved respirators can be found here: FDA Appendix A - Authorized Respirators.
American Dental Association infographic on understanding mask types can be found here: ADA Understanding Mask Types
MATERIAL:
- 46% non-woven polypropelene, 28% melt-blown fabric, 26% ES hot air cotton.
- Headband: Latex free
- Nose piece: Adjustable aluminum
FEATURES:
- Construction: Multi-layer filtration system of non-woven soft and breathable fibers.
- Headband style.
- All respirators manufactured after May 20th are affixed with an anti-fake sticker that can be used to verify authenticity.
SPECIFICATIONS:
- KN95 mask is not intended for use as a surgical mask or to provide liquid barrier protection.
- For Guangzhou Powecom Labor Insurance Supplies Co., LTD. KN95 CE certificate, please click here.
All mask orders are fulfilled on an "as available" basis. For more information, please see Terms and Conditions for Product Purchases.